Consultancy
Specialized in food sciences and technology, quality control, quality assurance, green
chemistry studies plan, product development and safety, health, sustainability,
environment (she), specifically on continuous improvement, innovation and environment
waste management.
Santos S Consulting LLC

Antonio de Souza Santos Consulting LLC is specialized in Science and Food Technology, Quality and Productivity. The principal of the company is an industrial chemist and has an extensive experience in quality control. This includes quality control and development in agrochemical syntheses.
Since 1997, Mr. Santos has focused on the development of methods and analysis of nutritional supplements. The company has expertise in productive technical knowledge, science of its interactions, degradations, stabilities, and microbiological/physical and chemical quality control of vitamins and minerals.
He worked in several countries in Latin America with activities of Quality Control, Quality Assurance, Product Development and Safety, Health & Environment (SHE), specifically on continuous improvement, innovation, and environment and waste management at food companies.
The biotechnology consulting field provides different services to many sectors surrounding the health and well-being of human beings. One of the major sectors where biotech interferes are the dietary supplements field and food safety. Antonio de Souza Santos Consulting, LLC. has a comprehensive product portfolio focusing on dietary supplements and food safety.
QUALITY CONTROL

Quality Control is an essential requirement in any business sector, but it is especially critical in the food industry. The goals of quality food are to ensure that food products are safe and fit for human consumption, meet the requirements of customers of regulations, and are consistently high in quality.
According to the World Health Organization, Quality Control is a part of good manufacturing practices that is concerned with sampling, specifications, analytical testing, monitoring of all materials and environmental conditions in the factory and with releasing or rejecting materials for production use and finished products, using validated methods implemented by trained and experienced personnel.
The company possess extensive expertise in
UHPLC (Ultra High Performance Liquid Chromatography)


Training and application of Analytical method validation that is an important regulatory requirement in pharmaceutical analysis. High Performance Liquid Chromatography (HPLC/UHPLC) is commonly used as an analytical technique in developing and validating assay methods for drug products and drug substances
Control of minerals and heavy metals via ICP-MS (Inductively Coupled Plasma Mass Spectrometry).
PATHOGENS CONTROL
Control of pathogens (viruses, parasites, bacteria).

GREEN ANALYTICAL
GREEN ANALYTICAL CHEMISTRY
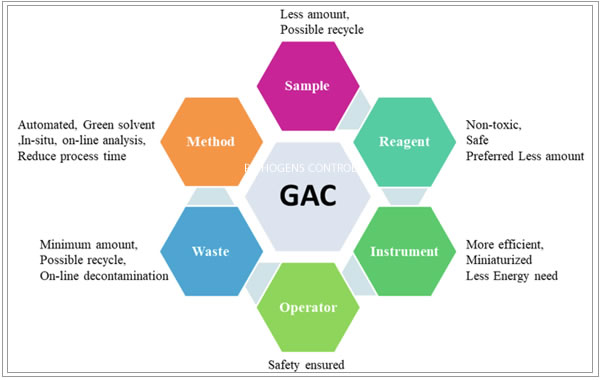
Green Analytical chemistry may be defined in light of the 12 principles of green chemistry as a collaboration of efforts to develop more clean or eco-friendly technologies characterized by elimination or at least minimizing emissions of pollutants into the environment without compromising efficiency even at low concentrations of analyte in samples with a complex matrix composition.
Scientific Literature Dealing With Green Analytical
Evolution of the scientific literature dealing with green analytical and chromatographic methods increased year after year due to the need to introduce new practice to comply with the principles of green chemistry for protection of analysts and the environment. This leads to the appearance of many searches for alternative techniques which have started to take a special importance recently aiming to reduce cost, time, complexity, extraction procedures, analyst exposure, energy, solvent, and waste production.
One of the principles of the environment-friendly approach to solutions used in analytical chemistry is to ensure the universality and availability of instrumental analytical techniques. As far as pharmaceutical analysis is concerned, the rule Reduce-Replace-Recycle (3Rs) appears to be the most relevant. In chromatographic analysis for the pharmaceutical industry, the principle of replacing and recycling is the one towards which the majority of researchers are oriented at the moment.
The Company Provides The Following Consulting Services
● Conduct studies using green analytical principles to identify changes in analytical methodologies and processes as well as use of solvents and reagents that are less harmful for people and the environment.
● Plan and implement the conversion of analytical methods from High-performance liquid chromatography (HPLC) to Ultra Performance Liquid Chromatography (UHPLC) and Ultra-High Performance liquid chromatography (UHPLC).
● Design analytical methods that eliminate or reduce solid waste and organic solvents.
● Elaborate and implement analytical techniques that reduce the time of analysis and thus diminishing the use of energy as well as organic solvents and its exposition to personnel.
● Design process using automation for direct analysis, such as the use of NIR infrared, which reduces waste and the need for organic solvents.
● Identify and implement analytical methods that eliminate the emission of gases and vapor.

ENVIRONMENT
ENVIRONMENT
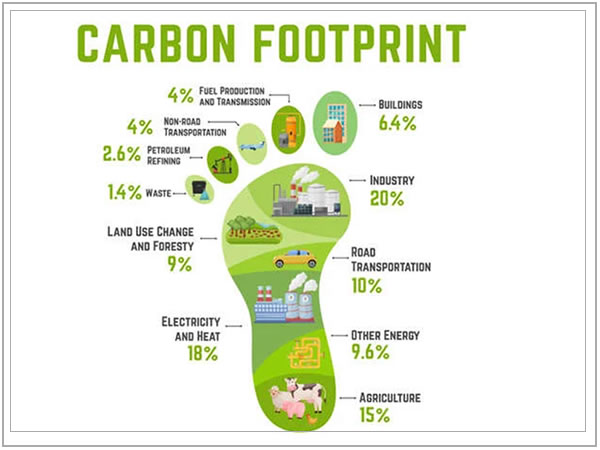
As we move into the future, decisions on food purchases will increasingly be influenced not only by price and quality, but also by social and environmental factors such as sustainability of technologies used for food production and processing and their environmental and health impacts. Growing consumer awareness about the impact of processing and production practices on the environment, the high energy consumption of certain processes, health impacts of the same technologies used in processing, and a heightened social and industrial consciousness to reduce the carbon-footprint are examples of factors influencing food choice. These factors have been made quite evident in the growing “buy local,”, “fair trade,” “certified organic,” trends. As a result, farmers and food manufacturers will increasingly be interested in identifying and using greener economically viable technologies for food production and processing.




The Company offers the following services
- Monitoring and disposal of batches rejected by QC
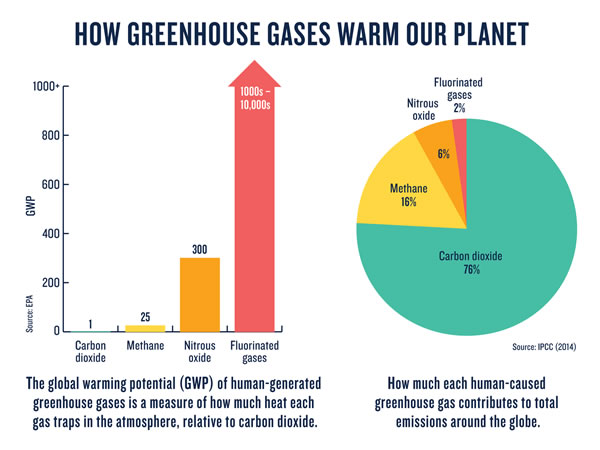
- Development and validation of analysis methods for the Measurement of Greenhouse Gases (GHG’s) with the purpose of evaluating both the environmental impact and the generation of carbon credits.
- Evaluation of waste generated by cleaning mixing tanks
- Water treatment of Reactor washing
- Assembly of specific modules for the treatment of toxic waste, prior to disposal in common treatment pipelines
- Comparison of waste treatment processes with greenhouse gas emissions, for carbon credit purposes

INDUSTRY 4.0
Industry 4.0 / Sustainability
Industry 4.0 is based on production with maximum efficiency, that is, with maximum efficiency addressing other productive factors in addition to reducing the cost of products that aim at maximum profitability. Parameters such as a renewable energy source, less impact on environmental resources and employees’ health now have as much weight as profits; thus, two requirements in which the company will act to:
- Recover of waste as by-products of other processes;
- andObtain quality data to support decision making based on quantitative information
Food industry is constantly improving their processing and production with caution in environmental impact. There are many areas where there is a need for more improvements. The idea is driven by society, nature via climate change, profit, management where smart decisions are needed. There are views from different interdisciplinary and interprofessional leaders and professionals, where different aspects need to be considered. Industry 4.0 is a way to improve production processes, to increase productivity, to reflect individual demands and short-term management wishes.


TRAINING
TRAINNING
The company provides several training sessions to its clients and partners in different subjects related to food safety.
GMP (Good Manufacturing Practices) are the practices required in order to conform to the guidelines recommended by agencies that control the authorization and licensing of the manufacture and sale of food and beverages, cosmetics, pharmaceutical products, dietary supplements, and medical devices.

HACCP
HACCP (Hazards analysis and critical control points) is a systematic preventive approach to food safety from biological, chemical, and physical hazards in production processes that can cause the finished product to be unsafe and designs measures to reduce these risks to a safe level.

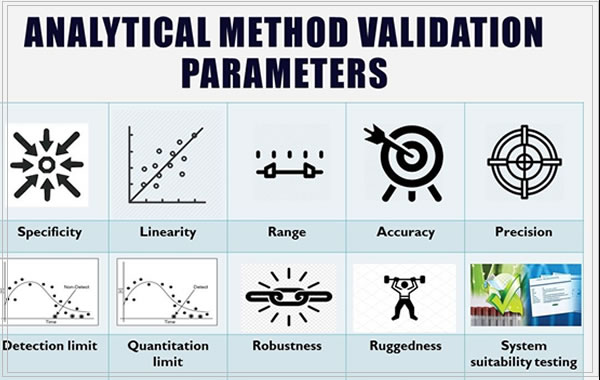
ANALYTICAL METHOD VALIDATION
Analytical method validation is an important regulatory requirement in pharmaceutical analysis.
The objective of validating an analytical method is to demonstrate that it is suitable for its intended purpose.
During validation, the characteristics applicable to the identification, impurities, assay procedures, controls, the frequency of use by different analysts in different labs, and applicable equipment use are included